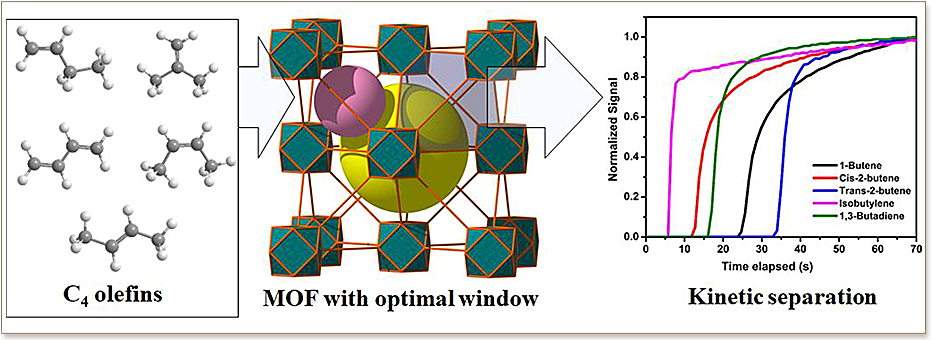
The separation of C4 olefin mixtures into pure components is one of the most challenging processes in chemical industries. Adsorption-based separation, using ultra-microporous materials, is considered as a viable alternative to reduce the operating cost of the currently employed but energy intensive distillation technique. The understanding of structural properties-relationships for C4 olefins separation using solid state materials is still lacking particularly for microporous materials with purely cage-based structures. Herein, the adsorptive separation of C4 olefins (butene isomers and 1,3-butadiene) by the microporous MOF, Y-fum-fcu-MOF, containing octahedral and tetrahedral cages with triangular pore aperture size (≈4.7 Å), is studied. The adsorption capacities of the MOF for C4 olefins were assessed by measuring the single component adsorption isotherms at 30 °C which led to very similar equilibrium adsorption uptakes at saturation for the components (excluding isobutylene). However, adsorption breakthrough curves collected at 25 °C and Pulse Gas Chromatography experiments, conducted to define the guest–host affinity at very low concentration (Henry’s region), evidenced kinetically driven separation of C4 olefins by Y-fum-fcu-MOF. In fact, a strong relation between pores window size, kinetic diameter of the components and their adsorption behavior was observed. The breakthrough capacity decreases at increasing molecular size, allowing the separation of cis- and trans-2-butene despite the quite similar adsorption enthalpies for these components (42.0 and 37.7 kJ/mol, respectively).