

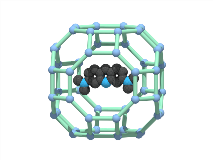
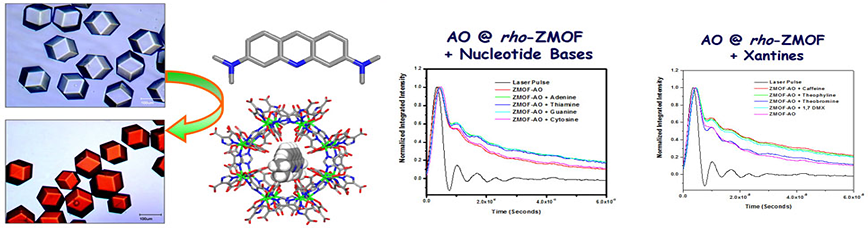
The anionic character and the large accessible voids of rho-ZMOF have allowed the full exchange of HPP cations with various organic and inorganic cations. This ability has led us to investigate the encapsulation of cationic probes (i.e. cridine chromophores, etc.) to sense neutral molecules. Acridine orange (AO, N,N,N9,N9-tetramethyl-3,6-acridinediamine) is smaller than the window dimensions of the rho-ZMOF (0.9 nm), consequently these molecules may freely diffuse into the larger cavities. Electrostatic interactions between the cationic AO and the negatively charged cavities of the rho-ZMOF preclude further diffusion of AO out of the cavities once encapsulated (anchored). Incubation of rho-ZMOF in an ethanolic solution of AO results in a red material with absorption and emission spectra similar to AO contained in n-heptane–sodium 1,4-bis(2-ethylhexyl)sulfosuccinate (AOT)–water reverse micelles. The extra-large cavities of rho-ZMOF can accommodate neutral molecules in addition to the cationic guest molecules that can only be exchanged with other cations. These features permit the exploration of this system to sense neutral molecules upon exchange with a cationic probe. Incubation of the AO–rho-ZMOF with solutions containing methyl xanthines or DNA nucleoside bases results in a change in both steady state emission and lifetime of the encapsulated AO that is dependent upon the nature of the second guest. These preliminary results demonstrate the ability of the ZMOF to serve as a (host–guest)-guest sensor where the ZMOF offers a periodic nanospace for fluorescent cations, which can then serve as the sensor unit.
© The Royal Society of Chemistry 2006.
Related
Publications