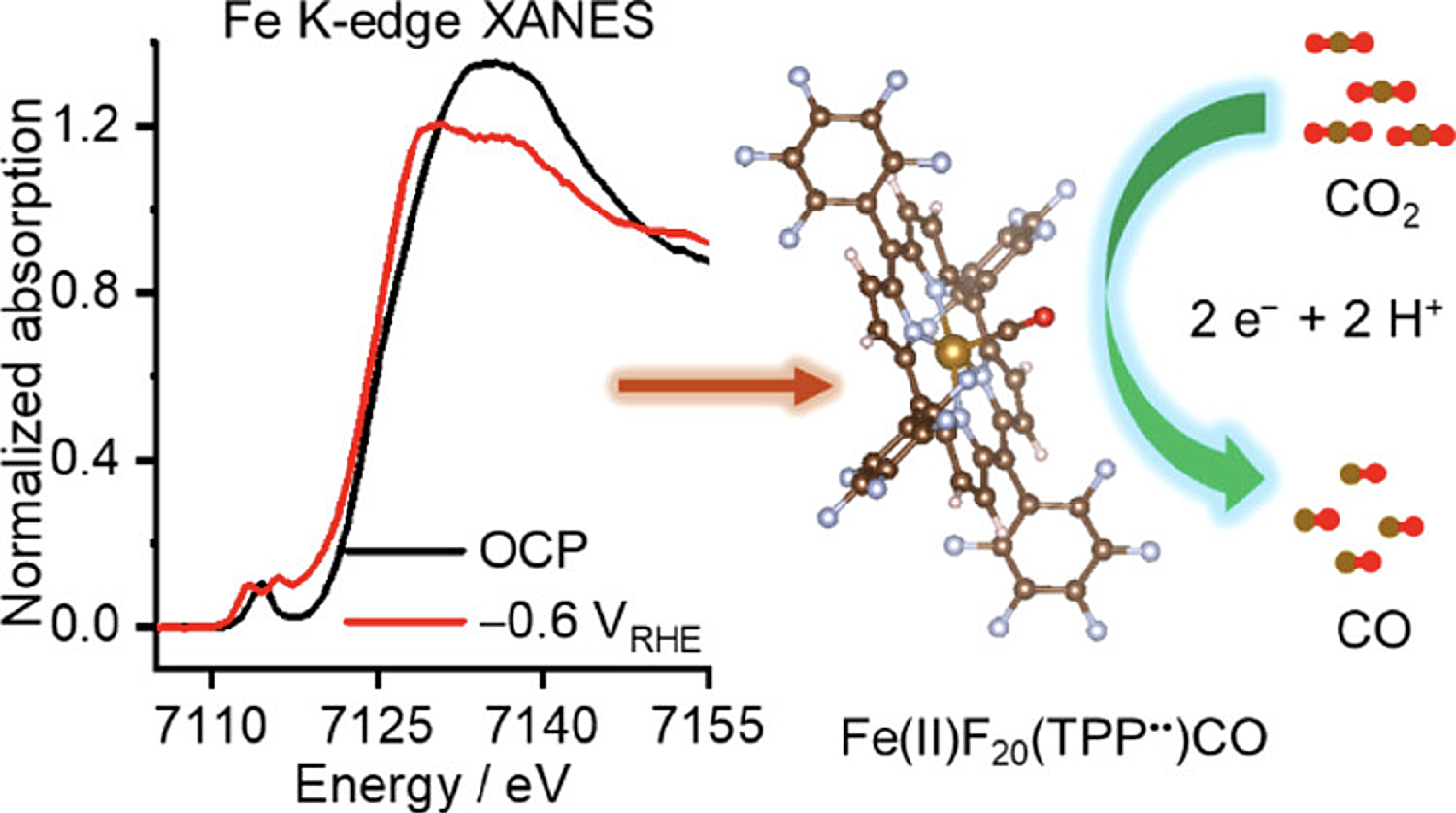
Iron porphyrin-based molecular catalysts can electrocatalyze CO2 reduction to CO at nearly 100% selectivity in water. Nevertheless, the associated active sites and reaction mechanisms remain debatable, impeding the establishment of design guidelines for effective catalysts. This study reports coupling in operando experiments and theoretical calculations for immobilized 5,10,15,20-tetrakis(pentafluorophenyl) porphyrin Fe(III) chloride (FeF20TPP) for electrocatalytic CO2 reduction in an aqueous phase. In operando UV–vis and X-ray absorption near-edge structure spectra indicated the persisting presence of Fe(II) species during the cathodic reaction, acting as catalytic sites that accommodate CO as Fe(II)–CO adducts. Consistently, the density functional calculations pointed out that the ligand-reduced state with oxidized Fe, namely, [Fe(II)F20(TPP•)]−, prevails in the catalytic cycle prior to the rate-controlling step. This work provides the conclusive representation related to the working states of Fe-based molecular catalysts under reaction conditions.