

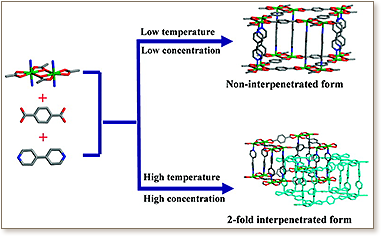
 A pillared framework formed from 4,4′-bipyridine (bipy) and sheets of dinuclear Cd2 nodes and 1,4-benzenedicarboxylic acid (bdc) linkers can be prepared in both interpenetrated and noninterpenetrated forms by simply varying the temperature or concentration of the reaction. These results demonstrate that higher temperatures and concentrations favor interpenetration over the corresponding noninterpenetrating structure, an observation that could have broad implications for controlling the synthesis of metal−organic materials.
A pillared framework formed from 4,4′-bipyridine (bipy) and sheets of dinuclear Cd2 nodes and 1,4-benzenedicarboxylic acid (bdc) linkers can be prepared in both interpenetrated and noninterpenetrated forms by simply varying the temperature or concentration of the reaction. These results demonstrate that higher temperatures and concentrations favor interpenetration over the corresponding noninterpenetrating structure, an observation that could have broad implications for controlling the synthesis of metal−organic materials.