

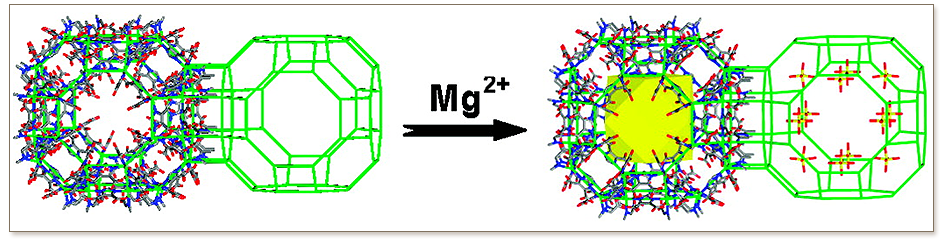
Zeolite-like metal−organic frameworks (ZMOFs) are anionic, have readily exchangeable extra-framework cations, and can be constructed with a variety of organic linkers. ZMOFs therefore can be regarded as an excellent platform for systematic studies of the effect(s) of various structural factors on H2 binding/interaction with porous metal−organic materials. We find that the enhanced binding of molecular hydrogen in ion-exchanged ZMOFs with an anionic framework is largely governed by the presence of the electrostatic field in the cavity, which is reflected by isosteric heats of adsorption in these compounds which are greater by as much as 50% relative to those in neutral MOFs. Direct contact of the sorbed hydrogen with the exchangeable cations is shown not to be possible in the explored systems thus far, as they retain their form as aqua complexes.