

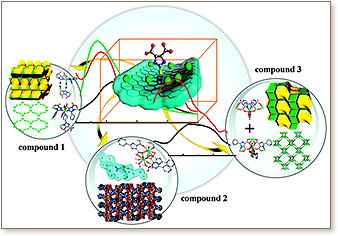
 Three novel metal−organic frameworks (MOFs), |(C3H7NO)2(H2O)|[Zn3(C10H5N3O4)3(C3H7NO)2] (1), |(H2O)5(H3O)(NO3)|[Nd2(C10H5N3O4)3(H2O)4] (2), and |(H2O)2|[Nd3(C10H5N3O4)3(C10H4N3O4)] (3), based on the T-shaped tripodal ligands 2-(pyridine-4-yl)-1H-4,5-imidazoledicarboxylic acid and 2-(pyridine-3-yl)-1H-4,5-imidazoledicarboxylic acid (H3PyImDC), have been constructed under solvo-/hydrothermal conditions. The diverse coordination modes of H3PyImDC ligands have afforded the assembly of three novel compounds. In compound 1, two oxygen atoms and three nitrogen atoms of the H3PyImDC ligand, a T-shaped linker, coordinate to two zinc centers to form a novel bbm net with two distinct channels along the [100] and [001] directions. In compound 2, H3PyImDC ligands coordinate to neodymium centers to form a ladder-like chain which then interacts with a water molecules chain via hydrogen-bondings to construct a 3D supermolecular structure. In compound 3, H3PyImDC ligands, a T-shaped linker, coordinate to neodymium centers to form a (3,6)-connected net with an ant topology. In compounds 1−3, the two H3PyImDC ligands exhibit different coordination modes with zinc and neodymium centers, which afforded the expected structural diversity. Additionally, all three compounds exhibit strong fluorescence emissions in the solid state at room temperature.
Three novel metal−organic frameworks (MOFs), |(C3H7NO)2(H2O)|[Zn3(C10H5N3O4)3(C3H7NO)2] (1), |(H2O)5(H3O)(NO3)|[Nd2(C10H5N3O4)3(H2O)4] (2), and |(H2O)2|[Nd3(C10H5N3O4)3(C10H4N3O4)] (3), based on the T-shaped tripodal ligands 2-(pyridine-4-yl)-1H-4,5-imidazoledicarboxylic acid and 2-(pyridine-3-yl)-1H-4,5-imidazoledicarboxylic acid (H3PyImDC), have been constructed under solvo-/hydrothermal conditions. The diverse coordination modes of H3PyImDC ligands have afforded the assembly of three novel compounds. In compound 1, two oxygen atoms and three nitrogen atoms of the H3PyImDC ligand, a T-shaped linker, coordinate to two zinc centers to form a novel bbm net with two distinct channels along the [100] and [001] directions. In compound 2, H3PyImDC ligands coordinate to neodymium centers to form a ladder-like chain which then interacts with a water molecules chain via hydrogen-bondings to construct a 3D supermolecular structure. In compound 3, H3PyImDC ligands, a T-shaped linker, coordinate to neodymium centers to form a (3,6)-connected net with an ant topology. In compounds 1−3, the two H3PyImDC ligands exhibit different coordination modes with zinc and neodymium centers, which afforded the expected structural diversity. Additionally, all three compounds exhibit strong fluorescence emissions in the solid state at room temperature.