

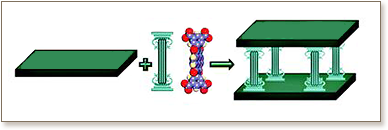
[Cu2(carboxylate)4] paddlewheel molecular building blocks, MBBs, are capable of generating square grid or Kagomé lattice supramolecular isomers when dicarboxylates such as 1,3-benzenedicarboxylate (1,3-bdc) and 1,4-benzenedicarboxylate (1,4-bdc) are exploited to link the paddlewheel MBBs. In this contribution we demonstrate that it is possible to use a solvent-free reaction (cocrystal controlled solid-state synthesis) to prepare a tetracarboxylic acid, H4BIPA-TC, formed by rigidly linking two 1,3-bdc moieties at the 5-position. BIPA-TC can pillar both square grid and Kagomé lattice supramolecular isomers, thereby generating nets that exhibit lvt or nbo topologies, respectively.