

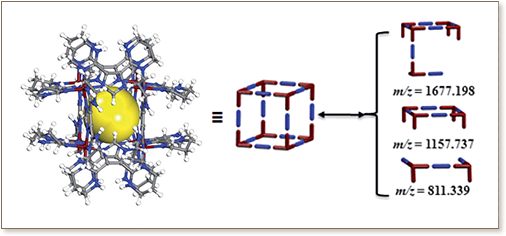
 Systematic studies were conducted to gain a better understanding of the metal-organic cubes (MOCs) directed assembly and their crystallization under predetermined reaction conditions, i.e. charge and size of metal ions, solvent type, counter anions, pH, and temperature. Four novel metal-organic materials are constructed via solvothermal reactions of different metal ions and 2,2′-(1H-imidazole-4,5-diyl)di-1,4,5,6-tetrahydropyrimidine, namely [Co 8(C 11N 6H 15) 12]Cl 12·4H 2O (1), [Ni 4(C 11N 6H 15) 4](NO 3) 4· 4DMF (2), {Cd(C 11N 6H 15)(NO 3) ·DMF} n (3), and [In 8(C 11N 6H 15) 12](NO 3) 12· 4H 2O (4). In addition, syntheses and crystal structures for compounds 1(a-f), constructed under deliberately modified reaction conditions of 1, are reported. In compounds 1(a-f), the Co III-based cationic MOCs crystallize in various packing arrangements in the presence of different counter-ions. Discrete MOCs retain their structural integrity, when crystalline solid was dissolved in water, under various pH (2.03-8.07) and temperatures (298-333 K), as confirmed by solution NMR studies. The assembly of the discrete MOC, from its basic molecular building blocks under mild reaction conditions, is demonstrated and monitored through solution NMR and UV-vis studies.
Systematic studies were conducted to gain a better understanding of the metal-organic cubes (MOCs) directed assembly and their crystallization under predetermined reaction conditions, i.e. charge and size of metal ions, solvent type, counter anions, pH, and temperature. Four novel metal-organic materials are constructed via solvothermal reactions of different metal ions and 2,2′-(1H-imidazole-4,5-diyl)di-1,4,5,6-tetrahydropyrimidine, namely [Co 8(C 11N 6H 15) 12]Cl 12·4H 2O (1), [Ni 4(C 11N 6H 15) 4](NO 3) 4· 4DMF (2), {Cd(C 11N 6H 15)(NO 3) ·DMF} n (3), and [In 8(C 11N 6H 15) 12](NO 3) 12· 4H 2O (4). In addition, syntheses and crystal structures for compounds 1(a-f), constructed under deliberately modified reaction conditions of 1, are reported. In compounds 1(a-f), the Co III-based cationic MOCs crystallize in various packing arrangements in the presence of different counter-ions. Discrete MOCs retain their structural integrity, when crystalline solid was dissolved in water, under various pH (2.03-8.07) and temperatures (298-333 K), as confirmed by solution NMR studies. The assembly of the discrete MOC, from its basic molecular building blocks under mild reaction conditions, is demonstrated and monitored through solution NMR and UV-vis studies.