

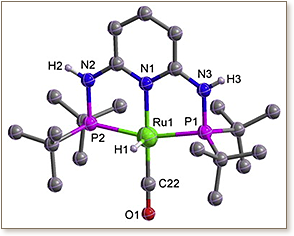
 A dearomatized complex [RuH(PN 3P)(CO)] (PN 3PN, N′-bis(di-tert-butylphosphino)-2,6-diaminopyridine) (3) was prepared by reaction of the aromatic complex [RuH(Cl)(PN 3P)(CO)] (2) with t-BuOK in THF. Further treatment of 3 with formic acid led to the formation of a rearomatized complex (4). These new complexes were fully characterized and the molecular structure of complex 4 was further confirmed by X-ray crystallography. In complex 4, a distorted square-pyramidal geometry around the ruthenium center was observed, with the CO ligand trans to the pyridinic nitrogen atom and the hydride located in the apical position. The dearomatized complex 3 displays efficient catalytic activity for hydrogen transfer of ketones in isopropanol.
A dearomatized complex [RuH(PN 3P)(CO)] (PN 3PN, N′-bis(di-tert-butylphosphino)-2,6-diaminopyridine) (3) was prepared by reaction of the aromatic complex [RuH(Cl)(PN 3P)(CO)] (2) with t-BuOK in THF. Further treatment of 3 with formic acid led to the formation of a rearomatized complex (4). These new complexes were fully characterized and the molecular structure of complex 4 was further confirmed by X-ray crystallography. In complex 4, a distorted square-pyramidal geometry around the ruthenium center was observed, with the CO ligand trans to the pyridinic nitrogen atom and the hydride located in the apical position. The dearomatized complex 3 displays efficient catalytic activity for hydrogen transfer of ketones in isopropanol.