

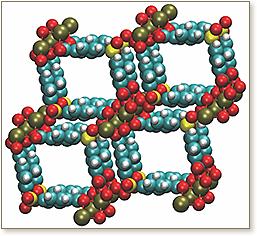
 A high fidelity molecular model is developed for a metal-organic framework (MOF) with narrow (approximately 7.3 Å ) nearly square channels. MOF potential models, both with and neglecting explicit polarization, are constructed. Atomic partial point charges for simulation are derived from both fragment-based and fully periodic electronic structure calculations. The molecular models are designed to accurately predict and retrodict material gas sorption properties while assessing the role of induction for molecular packing in highly restricted spaces. Thus, the MOF is assayed via grand canonical Monte Carlo (GCMC) for its potential in hydrogen storage. The confining channels are found to typically accommodate between two to three hydrogen molecules in close proximity to the MOF framework at or near saturation pressures. Further, the net attractive potential energy interactions are dominated by van der Waals interactions in the highly polar MOF - induction changes the structure of the sorbed hydrogen but not the MOF storage capacity. Thus, narrow channels, while providing reasonably promising isosteric heat values, are not the best choice of topology for gas sorption applications from both a molecular and gravimetric perspective.
A high fidelity molecular model is developed for a metal-organic framework (MOF) with narrow (approximately 7.3 Å ) nearly square channels. MOF potential models, both with and neglecting explicit polarization, are constructed. Atomic partial point charges for simulation are derived from both fragment-based and fully periodic electronic structure calculations. The molecular models are designed to accurately predict and retrodict material gas sorption properties while assessing the role of induction for molecular packing in highly restricted spaces. Thus, the MOF is assayed via grand canonical Monte Carlo (GCMC) for its potential in hydrogen storage. The confining channels are found to typically accommodate between two to three hydrogen molecules in close proximity to the MOF framework at or near saturation pressures. Further, the net attractive potential energy interactions are dominated by van der Waals interactions in the highly polar MOF - induction changes the structure of the sorbed hydrogen but not the MOF storage capacity. Thus, narrow channels, while providing reasonably promising isosteric heat values, are not the best choice of topology for gas sorption applications from both a molecular and gravimetric perspective.