

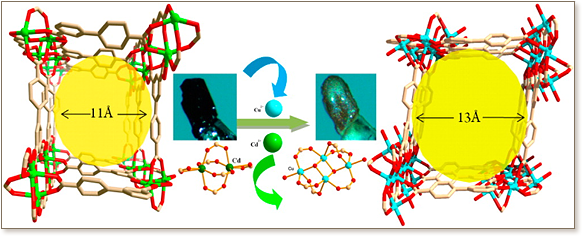
 When immersed in solutions containing Cu(II) cations, the microporous metal-organic material P11 ([Cd-4(BPT)(4)]center dot[Cd(C44H36N8)(S)]center dot[S], BPT = bi-phenyl-3,4',5-tricarboxylate) undergoes a transformation of its [Cd-2(COO)(6)](2-) molecular building blocks (MBBs) into novel tetranuclear [Cu4X2(COO)(6)(S)(2)] MBBs to form P11-Cu. The transformation occurs in single-crystal to single-crystal fashion, and its stepwise mechanism was studied by varying the Cd2+/Cu2+ ratio of the solution in which crystals of P11 were immersed. P11-16/1 (Cd in framework retained, Cd in encapsulated porphyrins exchanged) and other intermediate phases were thereby isolated and structurally characterized. P11-16/1 and P11-Cu retain the microporosity of P11, and the relatively larger MBBs in P11-Cu permit a 20% unit cell expansion and afford a higher surface area and a larger pore size.
When immersed in solutions containing Cu(II) cations, the microporous metal-organic material P11 ([Cd-4(BPT)(4)]center dot[Cd(C44H36N8)(S)]center dot[S], BPT = bi-phenyl-3,4',5-tricarboxylate) undergoes a transformation of its [Cd-2(COO)(6)](2-) molecular building blocks (MBBs) into novel tetranuclear [Cu4X2(COO)(6)(S)(2)] MBBs to form P11-Cu. The transformation occurs in single-crystal to single-crystal fashion, and its stepwise mechanism was studied by varying the Cd2+/Cu2+ ratio of the solution in which crystals of P11 were immersed. P11-16/1 (Cd in framework retained, Cd in encapsulated porphyrins exchanged) and other intermediate phases were thereby isolated and structurally characterized. P11-16/1 and P11-Cu retain the microporosity of P11, and the relatively larger MBBs in P11-Cu permit a 20% unit cell expansion and afford a higher surface area and a larger pore size.