

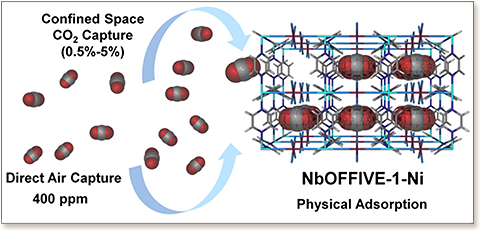
 The development of functional solid-state materials for carbon capture at low carbon dioxide (CO2) concentrations, namely, from confined spaces (<0.5%) and in particular from air (400 ppm), is of prime importance with respect to energy and environment sustainability. Herein, we report the deliberate construction of a hydrolytically stable fluorinated metal–organic framework (MOF), NbOFFIVE-1-Ni, with the appropriate pore system (size, shape, and functionality), ideal for the effective and energy-efficient removal of trace carbon dioxide. Markedly, the CO2-selective NbOFFIVE-1-Ni exhibits the highest CO2 gravimetric and volumetric uptake (ca. 1.3 mmol/g and 51.4 cm3 (STP) cm–3) for a physical adsorbent at 400 ppm of CO2 and 298 K. Practically, NbOFFIVE-1-Ni offers the complete CO2 desorption at 328 K under vacuum with an associated moderate energy input of 54 kJ/mol, typical for the full CO2 desorption in conventional physical adsorbents but considerably lower than chemical sorbents. Noticeably, the contracted square-like channels, affording the close proximity of the fluorine centers, permitted the enhancement of the CO2–framework interactions and subsequently the attainment of an unprecedented CO2 selectivity at very low CO2 concentrations. The precise localization of the adsorbed CO2 at the vicinity of the periodically aligned fluorine centers, promoting the selective adsorption of CO2, is evidenced by the single-crystal X-ray diffraction study on NbOFFIVE-1-Ni hosting CO2 molecules. Cyclic CO2/N2 mixed-gas column breakthrough experiments under dry and humid conditions corroborate the excellent CO2 selectivity under practical carbon capture conditions. Pertinently, the notable hydrolytic stability positions NbOFFIVE-1-Ni as the new benchmark adsorbent for direct air capture and CO2 removal from confined spaces.
The development of functional solid-state materials for carbon capture at low carbon dioxide (CO2) concentrations, namely, from confined spaces (<0.5%) and in particular from air (400 ppm), is of prime importance with respect to energy and environment sustainability. Herein, we report the deliberate construction of a hydrolytically stable fluorinated metal–organic framework (MOF), NbOFFIVE-1-Ni, with the appropriate pore system (size, shape, and functionality), ideal for the effective and energy-efficient removal of trace carbon dioxide. Markedly, the CO2-selective NbOFFIVE-1-Ni exhibits the highest CO2 gravimetric and volumetric uptake (ca. 1.3 mmol/g and 51.4 cm3 (STP) cm–3) for a physical adsorbent at 400 ppm of CO2 and 298 K. Practically, NbOFFIVE-1-Ni offers the complete CO2 desorption at 328 K under vacuum with an associated moderate energy input of 54 kJ/mol, typical for the full CO2 desorption in conventional physical adsorbents but considerably lower than chemical sorbents. Noticeably, the contracted square-like channels, affording the close proximity of the fluorine centers, permitted the enhancement of the CO2–framework interactions and subsequently the attainment of an unprecedented CO2 selectivity at very low CO2 concentrations. The precise localization of the adsorbed CO2 at the vicinity of the periodically aligned fluorine centers, promoting the selective adsorption of CO2, is evidenced by the single-crystal X-ray diffraction study on NbOFFIVE-1-Ni hosting CO2 molecules. Cyclic CO2/N2 mixed-gas column breakthrough experiments under dry and humid conditions corroborate the excellent CO2 selectivity under practical carbon capture conditions. Pertinently, the notable hydrolytic stability positions NbOFFIVE-1-Ni as the new benchmark adsorbent for direct air capture and CO2 removal from confined spaces.