

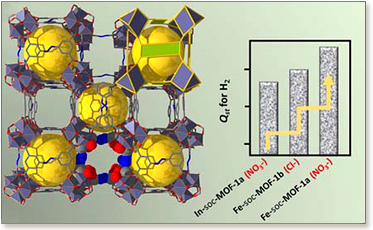
 We report on the synthesis and gas adsorption properties (i.e., Ar and H2) of four robust 3-periodic metal–organic frameworks (MOFs) having the targeted soc topology. These cationic MOFs are isostructural to the parent indium-based MOF, In-soc-MOF-1a (for NO3–), previously reported by us, and likewise are constructed from the assembly of rigid μ3-oxygen-centered trinuclear metal carboxylate clusters, [M3O(O2C−)6], where M = In3+ or Fe3+. Each inorganic trinuclear molecular building block (MBB), generated in situ, is bridged by six 3,3′,5,5′-azobenzenetetracarboxylate (ABTC4–) ligands to give the extended (4,6)-connected MOF, soc-MOF. In our previous work, we confirmed that the parent soc-MOF, i.e., In-soc-MOF-1a, possesses unique structural characteristics (e.g., vacant In binding sites and narrow pores with higher localized charge density), which led to exceptional hydrogen (H2) storage capabilities. Therefore, charged MOFs with soc topology can be viewed collectively as an ideal prototypical platform to examine the impact of specific structural parameters on H2–MOF interactions via systematic gas adsorption studies. We infer that enhanced binding of molecular H2 is primarily governed by the presence and type of vacant metal centers (i.e., Fe was shown to exhibit stronger H2–MOF interactions at low H2 loading compared to the In analogues). These findings are evident from the associated isosteric heat of adsorption (Qst) at low loadings and inelastic neutron scattering (INS) experiments of the rotational transitions of sorbed H2, as well as, temperature-programmed desorption (TPD) studies (for a select compound). The importance of localized charge density is also highlighted, where the extra-framework nitrate anions in the Fe-soc-MOF-1a (for NO3–) facilitate enhanced binding affinities as compared to the chloride analogue.
We report on the synthesis and gas adsorption properties (i.e., Ar and H2) of four robust 3-periodic metal–organic frameworks (MOFs) having the targeted soc topology. These cationic MOFs are isostructural to the parent indium-based MOF, In-soc-MOF-1a (for NO3–), previously reported by us, and likewise are constructed from the assembly of rigid μ3-oxygen-centered trinuclear metal carboxylate clusters, [M3O(O2C−)6], where M = In3+ or Fe3+. Each inorganic trinuclear molecular building block (MBB), generated in situ, is bridged by six 3,3′,5,5′-azobenzenetetracarboxylate (ABTC4–) ligands to give the extended (4,6)-connected MOF, soc-MOF. In our previous work, we confirmed that the parent soc-MOF, i.e., In-soc-MOF-1a, possesses unique structural characteristics (e.g., vacant In binding sites and narrow pores with higher localized charge density), which led to exceptional hydrogen (H2) storage capabilities. Therefore, charged MOFs with soc topology can be viewed collectively as an ideal prototypical platform to examine the impact of specific structural parameters on H2–MOF interactions via systematic gas adsorption studies. We infer that enhanced binding of molecular H2 is primarily governed by the presence and type of vacant metal centers (i.e., Fe was shown to exhibit stronger H2–MOF interactions at low H2 loading compared to the In analogues). These findings are evident from the associated isosteric heat of adsorption (Qst) at low loadings and inelastic neutron scattering (INS) experiments of the rotational transitions of sorbed H2, as well as, temperature-programmed desorption (TPD) studies (for a select compound). The importance of localized charge density is also highlighted, where the extra-framework nitrate anions in the Fe-soc-MOF-1a (for NO3–) facilitate enhanced binding affinities as compared to the chloride analogue.