

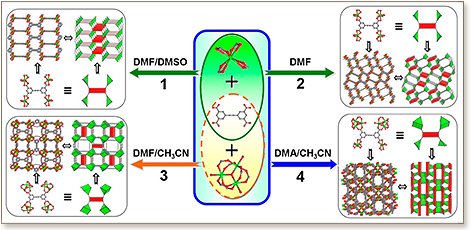
 Four metal-organic frameworks (MOFs) based on indium and a tetracarboxylate ligand have been synthesized through regulation of the reaction solvent conditions. The resultant compounds exhibited not only rich structural topologies (pts, soc, and unique topologies) but also interesting charge reversal framework features. By regulating the solvent, different building units (indium monomer, trimer) have been generated in situ, and they are connected with the ligand to form ionic frameworks 1-4, respectively. Among the synthesized four ionic frameworks, compounds 3 and 4 maintained keep their crystallinity upon heating temperature up to 300 °C and full removal of solvent guest molecules. Particularly, they also exhibit the charge reversal framework features (3 adopts an overall cationic framework, whereas 4 has an anionic framework). Both compounds 3 and 4 exhibit a notable significant uptake capacity for CO2 and H2; besides that, compounds 3 and 4 also offer excellent selective adsorption of CO2 over N2 and CH4.
Four metal-organic frameworks (MOFs) based on indium and a tetracarboxylate ligand have been synthesized through regulation of the reaction solvent conditions. The resultant compounds exhibited not only rich structural topologies (pts, soc, and unique topologies) but also interesting charge reversal framework features. By regulating the solvent, different building units (indium monomer, trimer) have been generated in situ, and they are connected with the ligand to form ionic frameworks 1-4, respectively. Among the synthesized four ionic frameworks, compounds 3 and 4 maintained keep their crystallinity upon heating temperature up to 300 °C and full removal of solvent guest molecules. Particularly, they also exhibit the charge reversal framework features (3 adopts an overall cationic framework, whereas 4 has an anionic framework). Both compounds 3 and 4 exhibit a notable significant uptake capacity for CO2 and H2; besides that, compounds 3 and 4 also offer excellent selective adsorption of CO2 over N2 and CH4.