

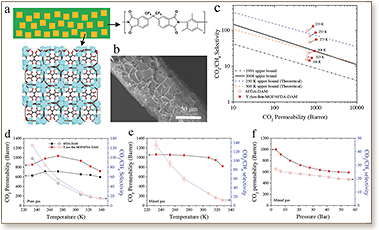
 Membrane‐based separations offer great potential for more sustainable and economical natural gas upgrading. Systematic studies of CO2/CH4 separation over a wide range of temperatures from 65 °C (338 K) to as low as −40 °C (233 K) reveals a favorable separation mechanism toward CO2 by incorporating Y‐fum‐fcu‐MOF as a filler in a 6FDA‐DAM polyimide membrane. Notably, the decrease of the temperature from 308 K down to 233 K affords an extremely high CO2/CH4 selectivity (≈130) for the hybrid Y‐fum‐fcu‐MOF/6FDA‐DAM membrane, about four‐fold enhancement, with an associated CO2permeability above 1000 barrers. At subambient temperatures, the pronounced CO2/CH4diffusion selectivity dominates the high permeation selectivity, and the enhanced CO2solubility promotes high CO2 permeability. The differences in adsorption enthalpy and activation enthalpy for diffusion between CO2 and CH4 produce the observed favorable CO2 permeation versus CH4. Insights into opportunities for using mixed‐matrix membrane‐based natural gas separations at extreme conditions are provided.
Membrane‐based separations offer great potential for more sustainable and economical natural gas upgrading. Systematic studies of CO2/CH4 separation over a wide range of temperatures from 65 °C (338 K) to as low as −40 °C (233 K) reveals a favorable separation mechanism toward CO2 by incorporating Y‐fum‐fcu‐MOF as a filler in a 6FDA‐DAM polyimide membrane. Notably, the decrease of the temperature from 308 K down to 233 K affords an extremely high CO2/CH4 selectivity (≈130) for the hybrid Y‐fum‐fcu‐MOF/6FDA‐DAM membrane, about four‐fold enhancement, with an associated CO2permeability above 1000 barrers. At subambient temperatures, the pronounced CO2/CH4diffusion selectivity dominates the high permeation selectivity, and the enhanced CO2solubility promotes high CO2 permeability. The differences in adsorption enthalpy and activation enthalpy for diffusion between CO2 and CH4 produce the observed favorable CO2 permeation versus CH4. Insights into opportunities for using mixed‐matrix membrane‐based natural gas separations at extreme conditions are provided.