

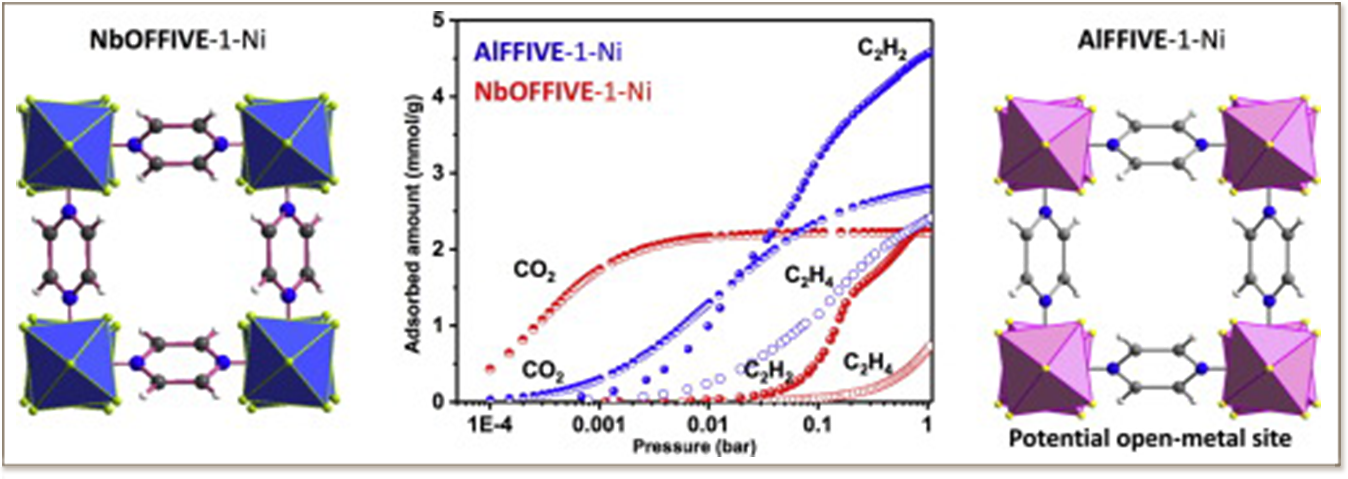
In this work, the reticular chemistry approach was implemented on a very stable ultra-microporous fluorinated MFFIVE-1-Ni MOFs to unveil the effect of subtle changes of the structure-employment of different fluorinated inorganic block on the adsorption of C2H2, C2H4 and CO2. A series of variable temperature single C2H2, C2H4 and CO2 adsorption isotherms and mixed gas adsorption column breakthrough experiments for different C2H2/C2H4 and CO2/C2H2 gas pair systems were carried out on the two isoreticular NbOFFIVE-1-Ni and AlFFIVE-1-Ni, containing respectively [NbOF5]2− and [AlF5]2− inorganic building blocks. The introduction of potential open metal site in a very confined pores led to favoring interaction of C2H2 but lowering the interaction with CO2, which resulted in enhancement of C2H2/C2H4 selectivity at low C2H2 concentration but a decrease in CO2/C2+selectivity. The comparison of the C2H2/C2H4 and CO2/C2H2 separation performances with the structures of the MFFIVE-1-Ni MOFs provides useful information to shed light on the relationship between the structural features of this MOF platform and C2H2/C2H4 and CO2/C2H2 separation properties. This is a critical step in the wanted rational discovery/design of materials with enhanced performances for C2H2 recovery from C2H4 and CO2 at different concentration and having different level of input energy for recycling.