

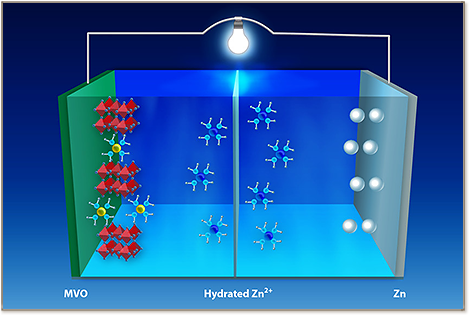
 The performance of chemically intercalated V2O5 was found to strongly depend on the interlayer spacing, which is related to the radius of hydrated metal ion, which can be readily tuned by using different intercalated metals. We report a layered Mg2+-intercalated V2O5 as the cathode material for aqueous ZIBs. The large radius of hydrated Mg2+ (∼4.3 Å, compared with 3.8 Å of commonly used Li+) results in an interlayer spacing as large as 13.4 Å (against 11.07 Å for Li+-intercalated V2O5), which allows efficient Zn2+ (de)insertion. As a result, the obtained porous Mg0.34V2O5·0.84H2O cathodes work in a wide potential window of 0.1 to 1.8 V versus Zn2+/Zn, and can deliver high capacities of 353 and 264 mA h –1 at current densities of 100 and 1000 mA g–1, respectively, along with long-term durability. Furthermore, the reversible Zn2+ (de)intercalation reaction mechanism is confirmed by multiple characterizations methods.
The performance of chemically intercalated V2O5 was found to strongly depend on the interlayer spacing, which is related to the radius of hydrated metal ion, which can be readily tuned by using different intercalated metals. We report a layered Mg2+-intercalated V2O5 as the cathode material for aqueous ZIBs. The large radius of hydrated Mg2+ (∼4.3 Å, compared with 3.8 Å of commonly used Li+) results in an interlayer spacing as large as 13.4 Å (against 11.07 Å for Li+-intercalated V2O5), which allows efficient Zn2+ (de)insertion. As a result, the obtained porous Mg0.34V2O5·0.84H2O cathodes work in a wide potential window of 0.1 to 1.8 V versus Zn2+/Zn, and can deliver high capacities of 353 and 264 mA h –1 at current densities of 100 and 1000 mA g–1, respectively, along with long-term durability. Furthermore, the reversible Zn2+ (de)intercalation reaction mechanism is confirmed by multiple characterizations methods.