

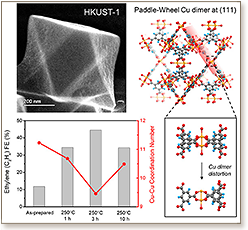
 The electrochemical carbon dioxide reduction reaction (CO2RR) produces diverse chemical species. Cu clusters with a judiciously controlled surface coordination number (CN) provide active sites that simultaneously optimize selectivity, activity, and efficiency for CO2RR. Here we report a strategy involving metal–organic framework (MOF)-regulated Cu cluster formation that shifts CO2electroreduction toward multiple-carbon product generation. Specifically, we promoted undercoordinated sites during the formation of Cu clusters by controlling the structure of the Cu dimer, the precursor for Cu clusters. We distorted the symmetric paddle-wheel Cu dimer secondary building block of HKUST-1 to an asymmetric motif by separating adjacent benzene tricarboxylate moieties using thermal treatment. By varying materials processing conditions, we modulated the asymmetric local atomic structure, oxidation state and bonding strain of Cu dimers. Using electron paramagnetic resonance (EPR) and in situ X-ray absorption spectroscopy (XAS) experiments, we observed the formation of Cu clusters with low CN from distorted Cu dimers in HKUST-1 during CO2 electroreduction. These exhibited 45% C2H4 faradaic efficiency (FE), a record for MOF-derived Cu cluster catalysts. A structure–activity relationship was established wherein the tuning of the Cu–Cu CN in Cu clusters determines the CO2RR selectivity.
The electrochemical carbon dioxide reduction reaction (CO2RR) produces diverse chemical species. Cu clusters with a judiciously controlled surface coordination number (CN) provide active sites that simultaneously optimize selectivity, activity, and efficiency for CO2RR. Here we report a strategy involving metal–organic framework (MOF)-regulated Cu cluster formation that shifts CO2electroreduction toward multiple-carbon product generation. Specifically, we promoted undercoordinated sites during the formation of Cu clusters by controlling the structure of the Cu dimer, the precursor for Cu clusters. We distorted the symmetric paddle-wheel Cu dimer secondary building block of HKUST-1 to an asymmetric motif by separating adjacent benzene tricarboxylate moieties using thermal treatment. By varying materials processing conditions, we modulated the asymmetric local atomic structure, oxidation state and bonding strain of Cu dimers. Using electron paramagnetic resonance (EPR) and in situ X-ray absorption spectroscopy (XAS) experiments, we observed the formation of Cu clusters with low CN from distorted Cu dimers in HKUST-1 during CO2 electroreduction. These exhibited 45% C2H4 faradaic efficiency (FE), a record for MOF-derived Cu cluster catalysts. A structure–activity relationship was established wherein the tuning of the Cu–Cu CN in Cu clusters determines the CO2RR selectivity.