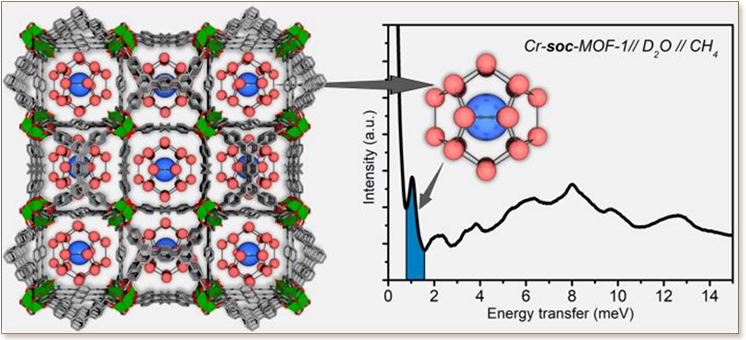
Porous MOFs capable of storing relatively high amount of dry methane (CH4) in adsorbed phase are largely explored, how-ever solid CH4 storage in confined pores of MOFs in the form of hydrates is yet to be discovered. Here we report a rational approach to form CH4 hydrates by taking advantage of the optimal pore confinement in relatively narrow cavities of hydro-lytically stable MOFs. Unprecedentedly, we were able to isolate methane hydrate (MH) nanocrystals with a sI structure en-capsulated inside MOF pores with an optimal cavity dimension. It was found, that confined nanocrystals require cavities slightly larger than the unit cell crystal size of MHs (1.2 nm), as exemplified in the experimental case study performed on Cr-soc-MOF-1 vs smaller cavities of Y-shp-MOF-5. Under these conditions, the excess amount of methane stored in the pores of Cr-soc-MOF-1 in the form of MH was found to be 50% larger than the corresponding dry adsorbed amount at 10 MPa. More importantly, the pressure gradient driving the CH4 storage/delivery process could be drastically reduced com-pared to the conventional CH4 adsorbed phase storage on the dry Cr-soc-MOF-1 (≤3 MPa vs. 10 MPa).